
Project
AIR-MUNE
In the NLAS project “Inversed air-liquid interface models for co-culture of airway epithelium with immune cells” (AIR-MUNE) researchers have developed innovative air-liquid interface models for bovine airway epithelial cells to study respiratory pathogens.
Respiratory tract infections are common in animals and humans and can cause severe disease. However, tools to study respiratory pathogensin vitroare limited. Primary cell or organoid-based airway epithelial cells (AECs) cultured at air-liquid interface are increasingly used for studying the pathogenesis of respiratory virus infections. These models consist of a well-differentiated stratified epithelium containing basal, mucus-forming and ciliated cells, with a top layer sealed by tight junctions. However, a major shortcoming of suchin vitromodels is the absence of immune cells, which are an important component of the submucosa of the respiratory epitheliumin vivo.
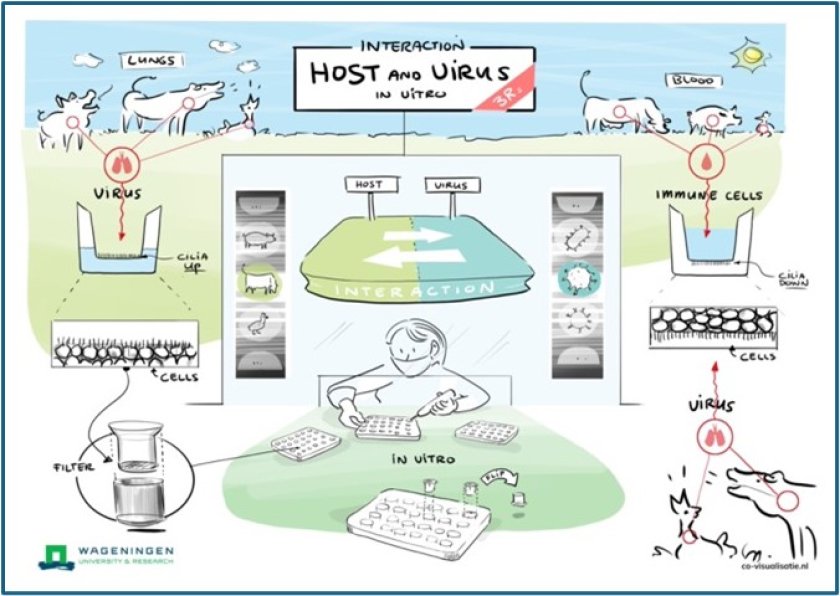
In recent years, “upside-down” air-liquid interface models have been described, in which epithelial cells are seeded on the bottom of a transwell filter with the basolateral side ‘up’. The advantage of this model is that immune cells can be added to the top compartment, where gravity will bring them in close contact with the basolateral side of the epithelium. When using transwell filters with a pore size of 3 µm, immune cells can pass the filter and migrate into the epithelium. This system has yielded promising results with human cells, but veterinary applications have not yet been developed.
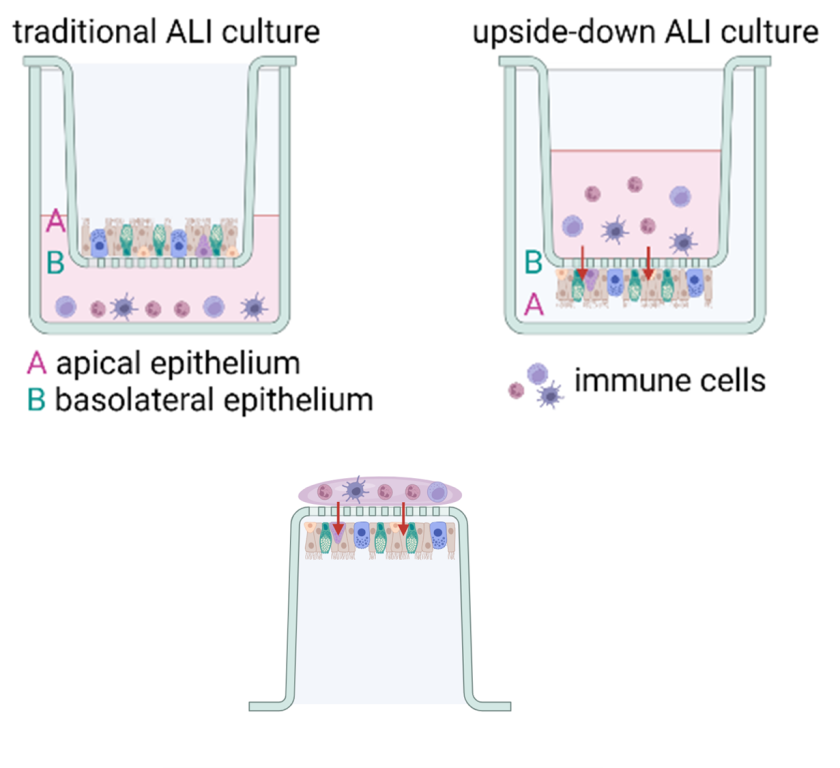
In the AIR-MUNE project we have developed upside-down models for bovine AECs in transwell filters with a pore size of 3 µm, and studied the impact of primary bovine neutrophils on infection with bovine respiratory syncytial virus (BRSV). Although we were successful in demonstrating migration of neutrophils into the epithelium, the epithelial cell culture at the bottom of the transwellfilter proved unstable. We therfore switched back to growing the epithelial cells at the top side of the transwellfilter, and added the neutrophils from below. Using this experimental design we were able to create a stable epithelial culture, infect the cells with BRSV and allow the neutrophils to infiltrate into the epithelium.
Wageningen Bioveterinary Research continues to invest in the development of new generation “complex cell systems” for in vitro investigation of inflammatory responses to respiratory infection, in an effort to contribute to the 3R goal of replacement, reduction and refinement in animal experimentation.