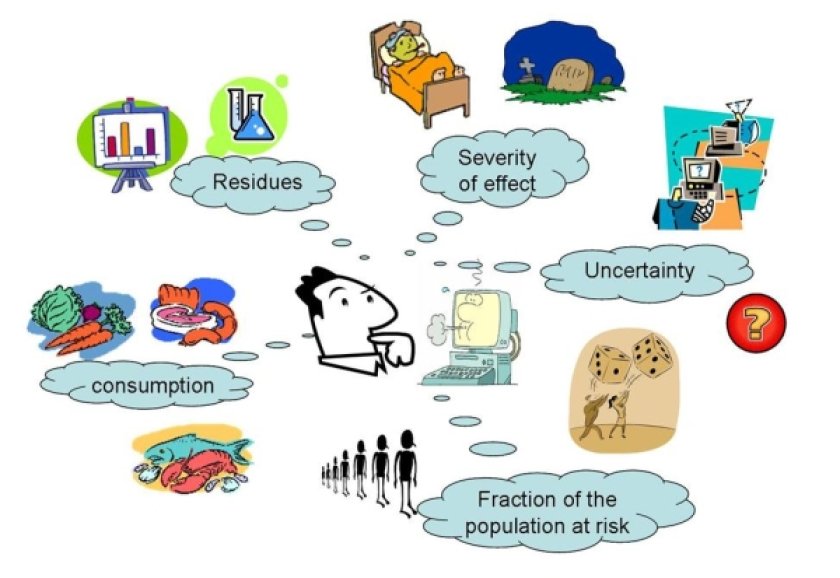
Work package 3
"Quantitative Risk Assessment of Combined Exposure to Food Contaminants and Natural Toxins."
The goal of this work package is to determine the health impact of human exposure to combinations of food contaminants (pesticides, mycotoxins) and natural toxins.
The ubiquitous presence of numerous contaminants in the environment and in food (such as pesticide residues and mycotoxins), even at relatively low levels, is of great public concern. However, risk assessment of exposure of humans to complex mixtures of food contaminants and natural toxins is still a great challenge.
Therefore, SAFE FOODS scientists working in Work package 3 (WP3) are developing new probabilistic approaches to evaluate cumulative effects. This is a largely unexplored area with many difficult challenges. For that reason, WP3 experts are focusing on simultaneous exposure to contaminants with the same working mechanism. Using models for probabilistic exposure, experts can assess potential health risks of food contaminants and/or natural toxins. Furthermore, a comparison is made of the risk of different chemicals and their possible interaction.

In addition, shortage in data regarding contamination of foods and limited access to databases is an important obstacle for exposure modelling. As a result, WP3 is also focusing on the development of larger and harmonised databases. Improved access to data regarding contamination of foods from the various production systems is an essential tool for exposure modelling.
WP3 experts are also working on evaluating uncertainties in risk assessment. Statistical routines are built into the quantitative risk models to distinguish risk uncertainty and risk variability. Moreover, analyses will be performed to quantify the level of uncertainty and variability. This has also implications for communication to risk managers and the consumer.
Last but not least, the input from this Work package will also be used to contribute to the development of a new integrated risk analysis approach for foods, which is the key task of Work package 6.
Questions
The primary questions addressed in this Work package are:
1. How to integrate exposure and effect modelling into quantitative risk modelling?
What does this mean? When addressing risks, you must take into account the following points:
- Exposure: levels of intake of a toxic compound in a population
- Effect: level that may bring about a toxic effect
- Quantifying risks : putting a figure to it
Quantitative risk modelling allows you:
- To quantify the margin between exposure and effect for different compounds
- To compare risks between different compounds
- To add risks of different compounds
2. How to set qualitative and quantitative criteria for comparing effects of various food chemicals and natural toxins to be used in combined exposure to food chemicals?
Since the availability of dose-response data is (always) limited, WP3 is reviewing the available toxicological data for their use in risk assessment of combined exposure (but also single exposure). Depending on this availability, Critical Effect Doses (CEDs) will be established for as many compounds as possible, using dose response modelling. WP3 will also review available toxicity classification systems to develop criteria in order to classify the toxicity of chemicals.
3. How to address uncertainty in various parts of the quantitative risk assessment?
A real challenge is to take the different types of uncertainty into account in the analyses, namely uncertainty in:
- data used in the assessment
- risk models available
- assumptions made on assessment variables
4. How to make better use of available consumption and residue databases?
An important deliverable for WP3 is the harmonisation of different EU food consumption and residue databases, in order to make the data appropriate for modelling. The harmonisation of food consumption databases includes the conversion of foods as eaten into raw agricultural commodities.
These databases will be used to quantify risks in probabilistic exposure assessments. In these experiments, special attention will be given to describing exposure in different European populations, including vulnerable groups (risk variability), as well as exposure at the Pan-European level.
Progress in Work package 3
1. Database Harmonisation
Residue and food consumption data are coming from national monitoring programmes and food consumption databases. Harmonisation of these data is crucial to guarantee comparability between partners and compatibility with the probabilistic modelling software. Therefore, SAFE FOODS has developed a Microsoft Access database to harmonise the compilation of residue data generated within the institutes of all WP3 partners.
In the beginning of the SAFE FOODS project, the WP3 partners attended a training in which they were instructed in the use of this database to organise their national residue and consumption data. In addition web servers have been prepared to connect residue and food consumption data present at different institutes to modelling software via Internet.
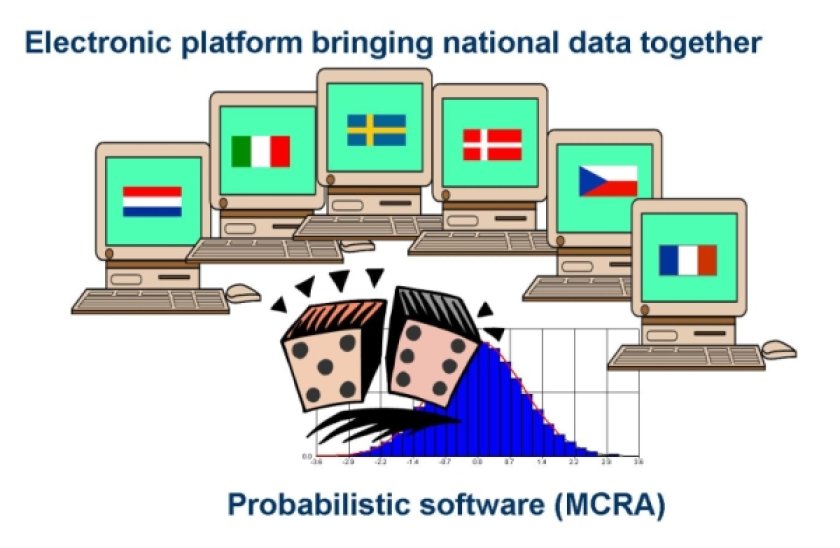
In total, residue data on 80 pesticides, 10 mycotoxins and 10 natural toxins have been collected from different countries. Furthermore, the food coding used in the different databases is now in the process of harmonisation to make it possible to perform Pan-European probabilistic exposure calculations.
Finally, WP3 has also assessed the quality of the available residue data. A position paper has been finalised on this subject addressing the availability, quality and access of data on food contaminants and natural toxins derived from different agricultural practices and breeding methods.
2. Probabilistic risk modelling
Probabilistic models have been released recently, but the technique is still young. The models are much more complicated than existing risk assessment procedures and are strongly data-dependent.
To test the quality of the harmonised national food consumption and residue data, probabilistic exposure calculations have been performed for single compounds (including pesticides, mycotoxins and natural toxins).
Currently, new exposure models are being built allowing for Pan-European modelling of exposure.
Effect modelling
Apart from exposure modelling, much work has been performed in the field of effect modelling. Dose-response analyses have been performed using the effect modelling software PROAST, resulting in the derivation of Critical Effect Doses for numerous pesticides, mycotoxins and natural toxins. A position paper has been prepared addressing data availability and data quality relevant for dose-response modelling.
Integration of effect and exposure, building risk models
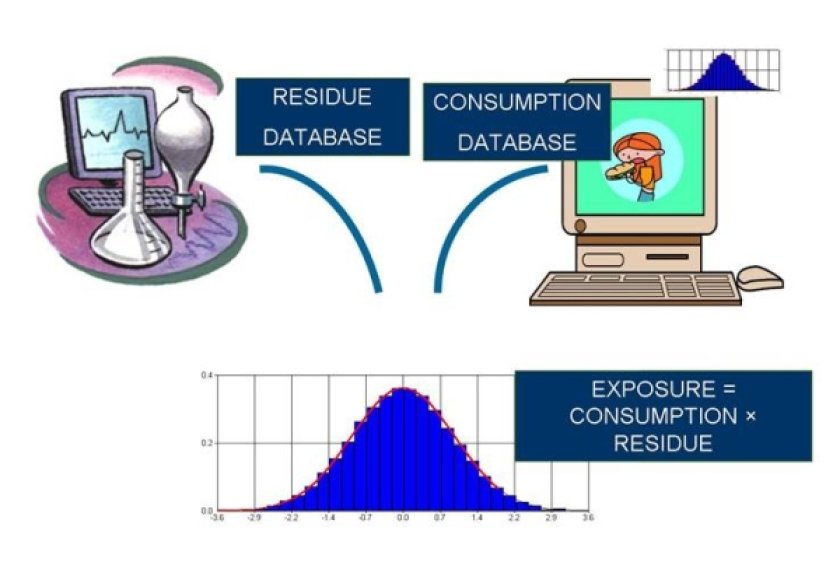
To integrate exposure and effect modelling for a new integrated probabilistic risk assessment, WP3 has developed a new statistical model. This probabilistic model is based on the Monte Carlo Risk Assessment (MCRA) software programme. A poster on MCRA: Probabilistic Dietary Risk Assessment can be found at the upper right of this page.
Within this model, residue, consumption and toxicology databases are used simultaneously to quantify the risk (margins of exposure and severity of toxic effects).
A manuscript entitled ‘Integration of probabilistic exposure assessment and probabilistic hazard characterization’ has been submitted, describing this model in detail. In this manuscript the Individual Margin of Exposure (IMoE) is proposed as a relevant instrument for integrated probabilistic risk assessment.
In the risk model, also a method is proposed to identify the major sources of uncertainty in the risk assessment. Statistical techniques, such as bootstrapping, have been used to estimate uncertainty in exposure and toxicity data. These routines are built into the quantitative risk models in order to distinguish uncertainty and variability.
Model uncertainty can be further explored, for example by comparing outcomes under different ways of modelling.
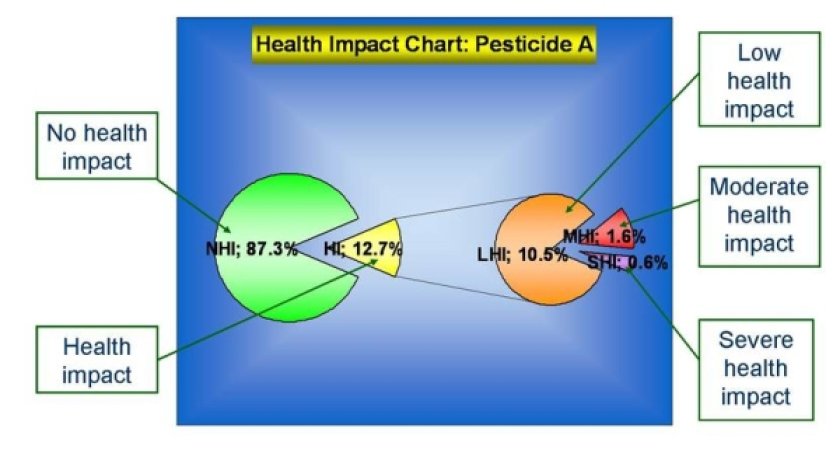
Toxicity classification of chemicals: taking severity of effect into account
Also a toxicity classification of chemicals will be included in the risk model. To this end, WP3 is developing criteria and methodologies for comparative hazard characterisation .
Among others, the following criteria have been reviewed on the basis of their applicability to compare health risks: Disability Adjusted Life Years (DALY), Life Cycle Assessment (LCA) and classification systems such as the one proposed by the International Life Science Institute ILSI. The conclusion was that none of the existing models is useful for SAFE FOODS purposes.
As a result, a new model will be developed for risk prioritization (‘health impact categorisation’). It will allow for the ranking of substances on their potential to induce health risks . This model will be based on the integrated probabilistic assessment of both effects and exposure and aims at accounting for effect type, effect size and fraction of population at risk.
Towards combined exposure
The newly established exposure and effect modelling, as well as the classification system for comparing toxic effects of different contaminants and natural toxins, will be integrated into a risk model to quantify effects of combined exposure.
A distinction will be made between compounds with the same and compounds with dissimilar modes of action. This model will be used to quantify risk from exposure to combined chemicals in different production systems. For chemicals where a dose-response curve is not relevant e.g. non-threshold chemicals, other forms of effect modelling will be explored.
Currently, WP3 works on hazardous compounds. However, the same methods can be adapted to benefits of chemical substances in foods. For that reason modeling of risk-benefit questions will also be addressed in SAFE FOODS (in addition to risk-risk comparisons).